
- Pagewriter tc cardiograph update#
- Pagewriter tc cardiograph manual#
- Pagewriter tc cardiograph software#
Proprietary Notice This document and the information contained in it is proprietary and confidential information of Philips Medical Systems ("Philips") and may not be reproduced, copied in whole or in part, adapted, modified, disclosed to others, or disseminated without the prior written permission of the Philips Legal Department. All other product names are the property of their respective owners.
Pagewriter tc cardiograph software#
This record will be updated as the status changes.1 PageWriter TC30 Cardiograph INSTRUCTIONS FOR USEĢ Notice About This Edition Published by Philips Medical Systems Printed in USA Publication number Edition History Edition 1, November 2009 Software Revision A and higher Edition 2, February 2010 Software Revision A and higher Copyright 2010 Koninklijke Philips Electronics N.V. Learn more about medical device recalls.Ģ Per FDA policy, recall cause determinations are subject to modification up to the point of termination of the recall.ģ The manufacturer has initiated the recall and not all products have been corrected or removed. The record is updated if the FDA identifies a violation and classifies the action as a recall, and it is updated for a final time when the recall is terminated. Foreign distribution worldwide.ġ A record in this database is created when a firm initiates a correction or removal action. The software will provide notification the customer when it is time to replace the battery, based on charge-discharge cycle count and the batteries state of health (SOH).ĭomestic distribution nationwide.
Pagewriter tc cardiograph update#
Users will be notified when the customer installable software update is available.

Philips plans to release a customer installable, software update for PageWriter TC cardiograph (TC20/30/50/70) that will provide alerts to assist users in managing the battery replacement cycle. Philips is instructing customers that once it is determined the battery is not in need of replacement, or once the battery is replaced, the PageWriter PC Cardiograph is safe to continuing using. ¿ Replace the battery if the number of cycles is greater than 300 and/or if the SOH is less than 80.Ĭustomers are instructed to complete and return the response card provided with the notice.
Pagewriter tc cardiograph manual#
¿ Promptly determine the number of cycles and the State of Health (SOH) on each of the affected Philips PageWriter TC cardiographs (TC20/30/50/70), as specified in the Service Manual Addendum. ¿ Carefully read the PageWriter TC Service Manual Addendum and to review this information with all staff members who are responsible for device management of the Philips PageWriter TC cardiographs Philips is asking customers to follow the ͊ction to be Taken by Customer/User section of the Urgent Medical Device Correction/Field Safety Notice This includes requesting that users: On December 8, 2018, Philips issued an Urgent Medical Device Correction letter to its customers, informing them of the product issue.
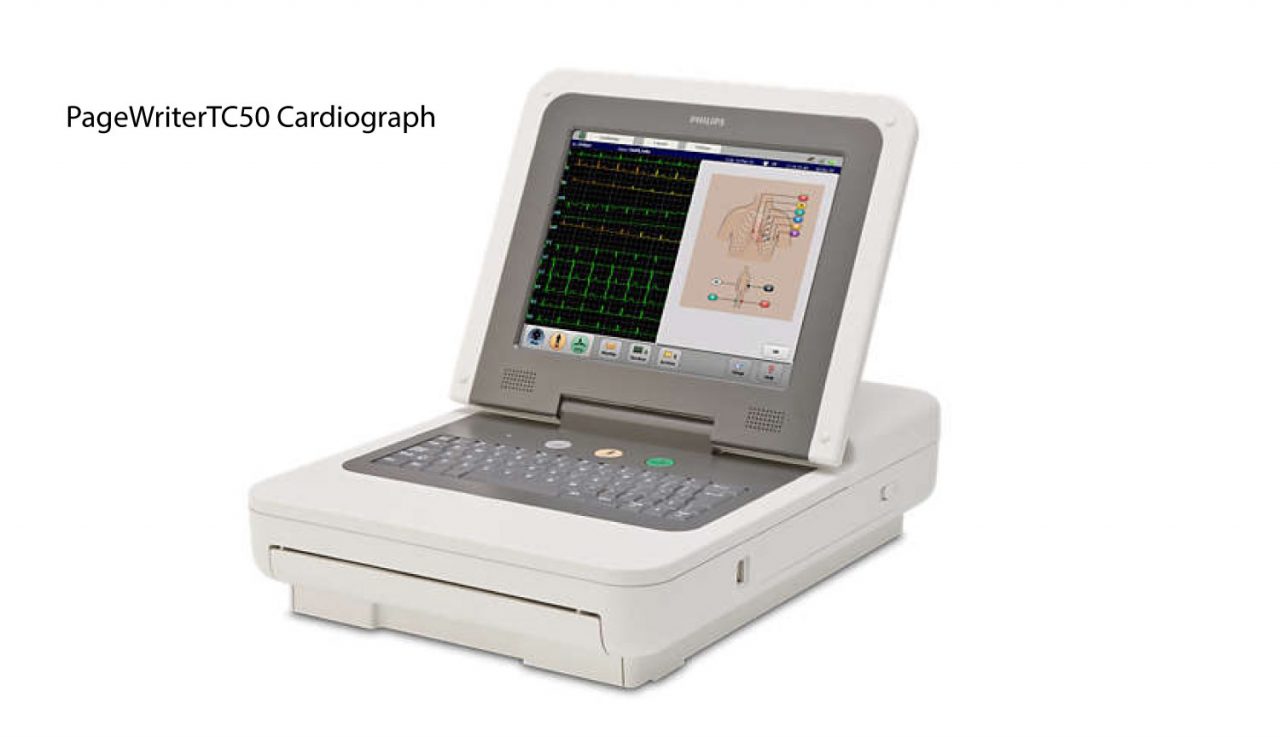
Philips PageWriter TC cardiograph batteries which have reached end of life may overheat, causing the case to melt and/or the device to ignite, which can cause injury to patient or nearby users. PageWriter TC70 w/o trolley Government Bundle, Product 860353, Software revisions up to and including A.07.05.22 to evaluate the electrocardiogram of adult and pediatric patients Class 2 Device Recall PageWriter TC70 w/o trolley Government Bundle


 0 kommentar(er)
0 kommentar(er)
